By Jennifer Kuzma*, Goodnight-NC GSK Foundation Distinguished Professor and Co-Director, Genetic Engineering and Society Center
*Note: The opinions expressed in this article are those of the author as an individual faculty member and should not be taken as a reflection of the views of the whole of the Genetic Engineering and Society Center or NC State University.
Note: The USDA SECURE act has been finalized. For Kuzma's recent description of the final rule, which differs from the draft rule, please see her presentation at our June 5th colloquium.
An example of the Trump administration’s emerging regulatory approach for biotechnology, a new draft rule to change USDA-Animal Plant Health and Inspection Service’s (APHIS) oversight of genetically engineered (GE) plants was published in the Federal Register on June 6, 2019, just a few days before the Executive Order on Modernizing the Regulatory Framework for Agricultural Biotechnology Products.1
In a previous blog,2 I described the way USDA-APHIS has regulated GE crops and discussed the Trump administration’s recall of proposed changes to GE crop oversight that were published late in the Obama administration. To recap, under the Coordinated Framework for the Regulation of Biotechnology (CFRB), USDA-APHIS has used its legal authorities under the Federal Plant Pest Act (1957) and updated Plant Protection Act (2000) to capture GE plants that are engineered using plant-pest DNA sequences. For example, in the first couple of decades of agricultural bioengineering, the primary method to put engineered genes into plants was to use DNA sequences from Agrobacterium, a bacterium that causes tumors on plants. These DNA sequences act as chaperones, helping to insert foreign DNA into plants. However, biotechnology outpaced our oversight system around the mid-2000s, and with newer transformation methods (such as gene guns) and precise gene editing methods (such as CRISPR), plant biotechnologists no longer had to use plant-pest sequences for genetic engineering. As such, dozens of GE crops, many that are gene-edited, were exempted from USDA-APHIS’s regulation from 2010 to the present, as determined by letter inquiries to USDA-APHIS from biotech developers, “Am I Regulated?” (AIR).3
In the final days of the Obama administration, in January 2017, USDA-APHIS proposed a change to its rules to include not only its plant-pest authorities but also its noxious weed authorities to regulate GE plants. This would have brought a “risk-based” scope to the regulation, and in my opinion, appropriately considered potential weed risks as a regulatory trigger. Demonstrated risks from the first generation of GE crops have centered around weediness (e.g. glyphosate-resistant weeds have resulted from GE herbicide-tolerant crops either due to cross-pollination with relatives or overuse of the herbicide). However, the 2017 changes would have likely expanded the number of GE crops for which the USDA-APHIS required field trials and petitions for deregulation in order to enter the market. In the new proposed rule (June 2019), USDA-APHIS states that “many (commentators) thought that the (2017) proposed requirements would be too burdensome and had the potential to stifle innovation.”4 As such, the agency withdrew the Obama proposed rule in November 2017.
Now the Trump administration is trying its own hand at regulatory reform for bioengineered crops.
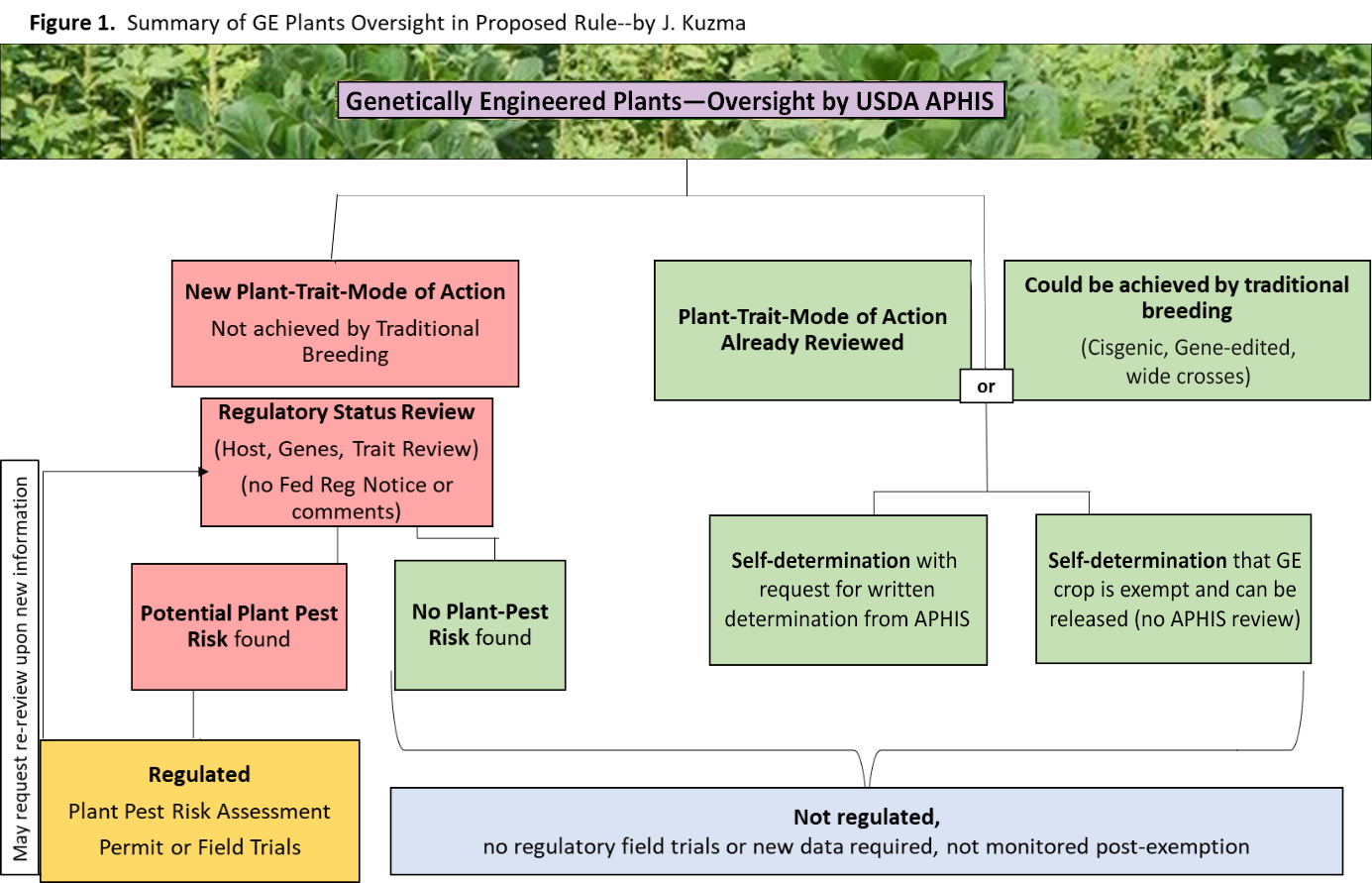
USDA-APHIS has proposed an oversight process for GE crops that appears to be a significant departure from the current one (Figure 1). Below are some of the key differences.5
Features of the New Proposed Rule
- The proposed rule shifts the USDA-APHIS regulatory trigger from “presence of plant-pest DNA sequences” in the GE crop to potential “plant-pest risk” from the GE crop.
- GE crops having the same host-trait-mechanism of action (MOA) combination that has been reviewed by USDA-APHIS in the past would be exempt from regulation and regulatory review.
Currently, regulation is based on a specific genetic modification, so that even if the same plant-trait-MOA exists, each insertion (or deletion) event is reviewed separately. Specific genetic engineering events would no longer trigger regulatory review if the plant host, the trait, and the MOA are similar to a GE crop reviewed in the past. - If a trait in a GE crop could have resulted from traditional breeding or is from a sexually compatible species (i.e. “cisgenic”), the GE crop would be exempt from regulatory review.
For example, gene edited crops resulting in cisgenic deletions or insertions (of any size) would be exempt if the gene-editing machinery (e.g. genes for CRISPR and guide RNA) is removed after the modification. Furthermore, the rule would exempt GE crops with genes that could have otherwise been introduced with traditional breeding techniques that enable “wide crosses” from “distantly related plants.” - Biotech developers will be able to self-determine whether a GE crop fits into one of the above categories for exemption with no USDA-APHIS review, or else request a letter of confirmation from USDA-APHIS that they are exempt.
- All GE plants that are not exempt will undergo a “regulatory status review” (RSR) for plant pest risk.
These will not require field or laboratory tests but rather will involve a review of existing literature and information. This review will consider the potential plant-pest risk given the type of DNA sequence change, host-plant’s geographic range and biology, environmental factors, and anticipated trait changes. USDA-APHIS describes the RSR process as “objective, rapid, and based on predetermined criteria” and having “functional similarity to the Am I Regulated? (AIR) Process.” Furthermore, APHIS states that there will be no public comment period or need to publish these decisions in the Federal Register, although a general database will be maintained of the plant-trait-MOA combinations having undergone RSR. From this review, if no plant-pest risk is anticipated, the GE plant would not be subject to regulation, could be released into the environment without restrictions, and could be moved in interstate commerce. - If there is a potential plant-pest risk that is indicated from the RSR, USDA-APHIS will conduct a full plant pest risk assessment (PPRA) to determine if the product is exempt, if a permit is needed to collect more data in field trials, or if the release of the GE plant should be limited in some way (e.g. to certain geographic regions).
These decisions would require publication in the Federal Register. This is the only category that constitutes formal premarket regulation by USDA and that has the potential to prohibit or limit environmental release and interstate movement. If regulation under a permit is required, a developer can request a re-review through the RSR process after data collection or upon new information about safety. - GE plants that have cleared the current AIR process would be grandfathered into exemption from regulation and remain unregulated under the proposed new rules, regardless of which category (cis- or transgenic) they fall into.
These include crops that have been gene-edited such as pennycress, camelina, mushrooms, potatoes, soybeans, corn, tomatoes, and wheat. It also includes crops with transgenes that have been engineered without the use of plant-pest sequences such as herbicide-tolerant grass.6 - “Noxious weed risk” as a regulatory trigger is still absent in the proposed rule (although if the GE crop poses a potential plant-pest risk, the PRRA and environmental assessment would include a consideration of weed-based risks).
Below I summarize what I believe are a few of the strengths and weaknesses of the proposed rule.
Strengths
- A shift from a “presence of plant-pest DNA sequences” as the trigger for USDA-APHIS regulation to a “plant-pest risk” trigger.
This shift bases regulatory scope on the “possibility of harm to plants resulting from introducing or disseminating a plant pest or from exacerbating the impact of a plant pest.” While still limited in the scope of risks considered, the new rule’s trigger is superior to the current regulatory trigger of GE plants that merely contain fragments of plant-pest DNA, which has nothing to do with environmental or agricultural risk. Regulation would be based on the plant-pest properties of the GE plant, not the mere presence of pest DNA. - A tiered approach, with flexibility in the oversight pathway based on past regulatory experience with that type of GE crop.
Forgoing a one-size-fits-all approach to GE crop regulation allows the USDA-APHIS to spend more time and limited resources on GE crops that are highly novel, may pose the highest risk, and for which environmental assessment is lacking. However, this flexibility could also be a detriment, making the process susceptible to political shifts, bias, and conflict-of-interest. In particular, the RSR decision-making process is a key point where agency bias in favor of GE product development could dominate.7
Weaknesses
- The USDA-APHIS’s proposed rule does not include a consideration of noxious weed risks as a trigger for premarket regulation of GE crops.
In the Environmental Impact Statement8 published to support the June 2019 draft rule, USDA-APHIS again considered the alternative of regulating GE crops based not only on plant-pest risks but also on noxious weed risks. The USDA-APHIS stated that one reason for doing this would be to promote the co-existence of farmers that grow GE crops with those that choose not to grow them (e.g. organic or non-GM farmers). The agency rejected this alternative, even though increased weed problems from the use of GE herbicide-tolerant crops have caused ecological and economic harm. For example, organic and non-GM farms have suffered from higher costs of weed removal and lost markets due to GE contamination.9 However, the EIS states “APHIS has never regulated based on economic impacts alone in the absence of any actual biological, chemical, or physical damage. This regulatory role would have been inconsistent with the Agency mission” and that “this Alternative was expected to increase regulatory costs to the agency and industry out of proportion to benefits” and “to decrease competitiveness of the U.S. biotech industry, and to stifle innovation.” Therefore, APHIS dismissed this Alternative from further consideration.
This seems inconsistent and unfair—essentially the USDA-APHIS is saying they cannot regulate solely because of economic harm to organic or non-GM farms, but they can decide not to regulate for economic reasons that favor the agricultural biotech industry (innovation and competitiveness). Furthermore, the new Executive Order clearly wants the agencies to consider how their regulatory actions affect markets and potentially harm industries10—but the mandate seems to only apply if the biotech industry is harmed, not the organic or non-GM industries. - The new approach will not require regulation, permitting, and field testing for most GE and gene edited plants.
The only GE crops that would undergo formal regulation are ones that show some sort of plant-pest risk from the RSR, which is based on published information for the non-GE counterpart. We know though that genetic changes, whether small or large, can increase toxicity of the plant to non-target species, weed potential, and other health or ecological risks.11 Biochemical pathways are finely tuned with multiple feedback loops and can be upset due to an increase or decrease in a native chemical (e.g. through cisgene editing) or the introduction of a foreign one. These unintended consequences are very difficult to predict. Without explicit safety testing on the exact genetic change in the plant, these risks are likely to go unnoticed. Even though the rule and Executive Order are purportedly “science-based,” forgoing scientific studies or field testing for most GE crops seems anti-science and precarious. - As in the Executive Order published along with the rule,12 the rhetoric is decidedly anti-regulation and pro-biotechnology. This attitude will do little to inspire public confidence.
The proposed rule is fraught with the same anti-regulatory bias as the EO. If the rule is implemented in favor of this bias, we can expect that very few, if any GE plants will require regulation. Regulatory status review or self-exemption will become the norm. With the speed and diversity that gene editing and other new biotechnologies bring to agriculture, GE plants of all kinds will soon dot the landscape. With no regulation comes no post-market monitoring for adverse effects. The public will likely believe that we have just opened a Pandora’s box. - Under the new rule, regulation would continue to be at least partially based on the process of genetic modification. Although this makes sense for practical reasons, it contradicts the claim that the “product” is the basis of regulation.
The USDA-APHIS rule purports to be based on product characteristics now (i.e. plant pest risk) and not the process by which it was made (e.g. via plant pest DNA sequences). However, the new framework is not completely risk-based, as it exempts cisgenic deletions or insertions (DNA from sexually compatible plants or wide crosses), which are, as a category, no more or less safe than transgenic insertions. For example, one can over-express a natural toxicant in a plant that makes it harmful to non-target insects and that can pose a significant ecological risk, whereas introduction of a gene from a distant species could be benign. USDA-APHIS uses a history of safe use and familiarity with conventional plant breeding to exempt cisgenic changes that could occur through these traditional genetic modification methods. This is a process-based exclusion, and thus the USDA-APHIS is contradicting its rationale for product-based regulation. An NASEM expert group13 suggested that with new genomic and bioinformatics analysis methods, all modified crop products—including those that are conventionally bred, GE, and gene edited— could be readily screened for increases in toxicants or decreases in nutrients. - The proposed oversight pathways will likely be less transparent than the current AIR, permit, notification, and deregulation oversight pathways.
The RSR and self-determination pathways do not provide for Federal Register notices and public comment procedures like permitting and deregulation petitions do now. It is unclear what information will be made public for RSRs or self-determinations. A lack of available information may exacerbate an already low level of trust and informed consent.14 Furthermore, allowing developers with a clear conflict of interest to self-determine their own regulatory pathway will likely provoke public skepticism, not confidence.
Summary
Overall, the new rule might improve the current situation in which developers of biotech crops are avoiding the use of plant-pest sequences to circumvent regulation. However, it is far from ideal. At a minimum, transparency should be a priority to engender public trust and allow for external scientific review. The new rule should require Federal Register publication and public comment not only for permits but also for RSRs. Also, self-determinations should be recorded by USDA APHIS and placed on a public website, like the current AIR process.
A public database of bioengineered crops going through all oversight pathways will allow for public tracking so that in the event of a mishap, incidents have a chance of being identified and attributed. Such adverse events occurred with the first generation of GE crops, including the comingling of human food with unapproved GE crop varieties and environmental contamination of non-GE crops with GE crops which affected international and national markets. For example, the presence of herbicide-tolerant GE wheat in the U.S. was reported recently. GE wheat has never been approved for marketing and has not been grown in field trials in the U.S for over 10 years, but its presence in Washington state was found the very same week as the proposed rule was released, causing concern among wheat growers that they will lose export markets.15
There is a need for USDA-APHIS to take a more careful approach to the regulation of the second generation of bioengineered crops in order to avoid the public and market failures of the first. As such, the proposed rule should be amended with assurances of transparency, outside scientific review, caution under uncertainty, and post-release monitoring.
2 https://ges.research.ncsu.edu/2017/11/politics-trumps-science-regulation-genetically-engineered-crops/
3 https://www.nature.com/articles/nbt.2143; https://www.nature.com/news/policy-reboot-the-debate-on-genetic-engineering-1.19506; https://www.aphis.usda.gov/aphis/ourfocus/biotechnology/am-i-regulated
4 https://www.aphis.usda.gov/aphis/ourfocus/biotechnology/biotech-rule-revision/2017_perdue_proposed_rule/340_2017_perdue_biotechreg
5 Note: due to space limitations, I do not discuss provisions in the proposed rule for pharmaceutical-containing GE crops, plant-incorporated-protectants in GE crops (also regulated by EPA), or plant-pests themselves (like GE insects that are plant pests). USDA discusses different pathways to regulate these products.
7 https://www.tandfonline.com/doi/full/10.1080/23299460.2017.1407912; https://philpapers.org/rec/MEGTRD